Unveiling The Dual Anticancer Role of Hilleria Latifolia (Phytolaccaceae): Use of Preliminary Bench-Top Bioassays in A Resource-Challenging Environment
Main Article Content
Abstract
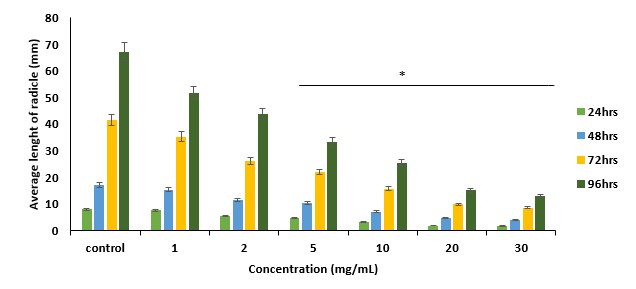
Medicinal plants remain a vital source for modern drug discovery. Research into plants traditionally used for tumour-related disorders is essential, considering the rising incidence of various forms of cancer. Hilleria latifolia is among the plants documented in traditional medicine for treating tumour-related ailments, but with limited scientific evidence. This study aimed to investigate the growth-inhibitory (antiproliferative) and cytotoxic potentials of the methanol extract of the whole plant of H. latifolia and its fractions using simple bench-top assay methods. Antiproliferative activity was conducted using guinea corn (Sorghum bicolor) at 1–30 mg/mL over 24-96 hours, while cytotoxicity was evaluated using tadpoles (Raniceps raninus) after 24 hours at 10–400 µg/mL. The crude methanol extract remarkably suppressed seed radicle proliferation in a concentration- and time-dependent manner, achieving 95.24% inhibition at 30 mg/mL. The aqueous and chloroform fractions exhibited 99.42% and 80.38% inhibition, respectively, at similar concentrations. In the cytotoxicity assay, the chloroform fraction and the crude methanol extract demonstrated the highest cytotoxicity, achieving 100% mortality at 200-400 μg/mL. The aqueous fraction achieved 93.3% mortality at 400 μg/mL. LC50 values of 159.26, 215.10, and 99.55 μg/mL were calculated for the methanol extract, the aqueous, and the chloroform fractions, respectively. Phytochemical evaluation confirmed the presence of condensed tannins, flavonoids, glycosides, alkaloids, and steroids, which have been linked to anticancer properties. These findings provide evidence supporting the ethnomedicinal use of H. latifolia for managing tumour-related conditions and suggest its potential as a lead in anticancer drug discovery, pending confirmation studies with established cancer cell lines.
Metrics
Article Details

This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License.
References
Iyasele JU, Uadia JO, Akhigbe IU, Jacob JN, Ogbeide OK. Physico-chemical properties, chemical composition, and antimicrobial activity of Adonidia merrillii kernel seed oil. Trop J Nat Prod Res. 2022;6(4):599-605. https://doi.org/10.26538/tjnpr/v6i4.22.
Adepoju AJ, Adepoju AA, Olawoore IT, Ayodele ET. Phytochemical profiling, antioxidant, and antidiabetic activities of defatted ethanol aerial extract of Heliotropium indicum: Insights from GC-MS and in vitro studies. Trop J Phytochem Pharm Sci. 2025; 4(8) 320 – 328. http://www.doi.org/10.26538/tjpps/v4i8.1.
Singh A, Sharma P, Kumar V. Therapeutic efficacy, and cost effectiveness of herbal drugs: A systematic review. J Herb Med. 2024;41:100688. https://doi.org/10.1016/j.hermed.2023.100688.
Falodun A, Okafor OI, Erharuyi O, Okugbo OT. Phytochemical Investigation and Antioxidant Activity Evaluation of Pyrenacantha staudtii (Icacinaceae) Leaf. Trop J Phytochem Pharm Sci. 2025;4(6):255-259. doi: https://doi.org/10.26538/tjpps/v4i6.2.
Newman DJ, Cragg GM. Natural products as sources of new drugs over the nearly four decades from 01/1981 to 09/2019. J Nat Prod. 2020;83(3):770-803. https://doi.org/10.1021/acs.jnatprod.9b01285.
Achan J, Talisuna AO, Erhart A, Yeka A, Tibenderana JK, Baliraine FN. Quinine, an old anti-malarial drug in a modern world: role in the treatment of malaria. Malar J. 2011;10:144. https://doi.org/10.1186/1475-2875-10-144
Brownstein MJ, Aghajanian GK. Codeine and its derivatives: an overview. Science. 1972;176(4038):1043–9. https://doi.org/10.1126/science.176.4038.1043
Noble RL. The discovery of the vinca alkaloids—chemotherapeutic agents against cancer. Biochem Cell Biol. 1990;68(12):1344–51. https://doi.org/10.1139/o90-197
Fabricant DS, Farnsworth NR. The value of plants used in traditional medicine for drug discovery. Environ Health Perspect. 2001;109(Suppl 1):69–75. https://doi.org/10.1289/ehp.01109s169
Atanasov AG, Zotchev SB, Dirsch VM, Supuran CT. Natural products in drug discovery: advances and opportunities. Nat Rev Drug Discov. 2021;20(3):200–16. https://doi.org/10.1038/s41573-020-00114-z.
Brown R, Short SC, Semple S. Cancer: A proposed definition for the 21st century. Semin Cancer Biol. 2023;92:1-5. https://doi.org/10.1016/j.semcancer.2023.07.001
World Health Organization (WHO). Cancer fact sheet. Geneva: WHO; 2025 [cited 2025 Sep 29]. Available from: https://www.who.int/news-room/fact-sheets/detail/cancer
Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A. Global cancer statistics 2022: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2024;74(3):229-63. https://doi.org/10.3322/caac.21834.
International Agency for Research on Cancer (IARC). Nigeria fact sheet: GLOBOCAN 2022. Lyon: IARC/WHO; 2022 [cited 2025 Sep 29]. Available from: https://gco.iarc.who.int/media/globocan/factsheets/populations/566-nigeria-fact-sheet.pdf
World Health Organization (WHO). Global cancer burden growing, amidst mounting need for services. Geneva: WHO; 2024 [cited 2025 Sep 29]. Available from: https://www.who.int/news/item/01-02-2024-global-cancer-burden-growing--amidst-mounting-need-for-services
Sung H, Ferlay J, Siegel RL, Laversanne M, Soerjomataram I, Jemal A. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2021;71(3):209-49. https://doi.org/10.3322/caac.21660.
Colditz GA, Wei EK. Preventability of cancer: The relative contributions of biologic and social and physical environmental determinants of cancer mortality. Annu Rev Public Health. 2012;33:137-56. https://doi.org/10.1146/annurev-publhealth-031811-124627.
Wu S, Powers S, Zhu W, Hannun YA. Substantial contribution of extrinsic risk factors to cancer development. Nature. 2018;529(7584):43-7. https://doi.org/10.1038/nature16166.
Bray F, Laversanne M, Weiderpass E, Soerjomataram I. The ever-increasing importance of cancer as an ever-increasingly important cause of premature death worldwide. Cancer. 2021;127(16):3029-30. https://doi.org/10.1002/cncr.33587.
National Cancer Institute (NCI). Cancer treatment types. Bethesda (MD): NCI/NIH; 2025 [cited 2025 Sep 29]. Available from: https://www.cancer.gov/about-cancer/treatment/types
Sung H, Anderson WF, Bray F, Ferlay J, Laversanne M, Gao YT. Global patterns in cancer incidence and mortality: epidemiologic insights. CA Cancer J Clin. 2022;72(5):333-362. doi: http://dx.doi.org/10.3322/caac.21728.
Mumtaz S, ul Haq I, Rauf A, Begum S, Ghulam S, Jahan N. Phytochemicals with potential anticancer activity from Moringa oleifera: An updated review. Saudi J Biol Sci. 2021;28(12):7338-50. https://doi.org/10.1016/j.sjbs.2021.08.069
Milugo TK, Omosa LK, Ochanda JO, Owuor BO, Oyugi JO, Wamunyokoli F. Antiproliferative and antimicrobial activities of compounds from Rauvolfia caffra (Apocynaceae). Nat Prod Res. 2016;30(17):2003-7. https://doi.org/10.1080/14786419.2015.1108514
Adedokun O, Sulaimon T, Mordi J, Adegboyega T, Oloyede O, Adeyemi A. Anticancer properties of Hymenocardia acida stem bark: Induction of apoptosis and inhibition of proliferation in human breast cancer cells. J Ethnopharmacol. 2022;285:114910. https://doi.org/10.1016/j.jep.2021.114910
Park M, Bae J, Lee DS. Antibacterial and anticancer activities of Zingiber officinale Roscoe. Molecules. 2014;19(5):6270-84. https://doi.org/10.3390/molecules19056270
Ajayi OS, Arowosegbe SM, Olawuni IJ. GC-MS characterization and bioactivity studies of aerial parts of Hilleria latifolia (Lam) extracts and fractions: antioxidant and antibacterial potentials. Ife J Sci. 2024;26(3):569-584. doi: http://dx.doi.org/10.4314/ijs.v26i3.2.
Woode E, Abotsi WKM, Boakye-Gyasi E. Antinociceptive effect of an ethanolic extract of the aerial parts of Hilleria latifolia (Lam.) H.Walter. Pharm Biol. 2011;49(7):646–53. https://doi.org/10.3109/13880209.2010.544421.
Abotsi WKM, Ainooson GK, Woode E. Anti-inflammatory and antioxidant effects of an ethanolic extract of the aerial parts of Hilleria latifolia (Lam.) H.Walter. Afr J Tradit Complement Altern Med. 2012;9(1):138–52. https://doi.org/10.4314/ajtcam.v9i1.18.
Johnson IS, Ettebong EO, Okokon JE. In vivo antiplasmodial activities of ethanolic leaf extract and fractions of Hilleria latifolia. J Med Plants Stud. 2017;5(4):118-22. Available from: https://www.plantsjournal.com/archives/2017/vol5issue4/PartB/5-4-3-547.pdf
Kew Science – Plants of the World Online. Hilleria latifolia (Lam.) H.Walter. Royal Botanic Gardens, Kew; 2025 [cited 2025 Sep 30]. Available from: https://powo.science.kew.org/taxon/urn:lsid:ipni.org:names:122647-2.
Schmelzer GH. Hilleria latifolia (Lam.) H.Walter. In: Schmelzer GH, Gurib-Fakim A, editors. Plant Resources of Tropical Africa 11(1): Medicinal plants 1. Wageningen: PROTA Foundation; 2007. p. 267–8. Available from: https://www.prota4u.org/database/protav8.asp?g=pe&p=Hilleria+latifolia.
Amponsah IK, Mensah AY, Otoo A. Pharmacognostic standardisation of Hilleria latifolia (Lam.) H.Walter. J Pharmacogn Phytochem. 2014;3(1):87–95. Available from: https://www.sciencedirect.com/science/article/pii/S2221169115301088
Odugbemi T. A textbook of medicinal plants from Nigeria. Lagos: University of Lagos Press; 2008. Available from: https://www.worldcat.org/oclc/299379681.
Iwu MM. Handbook of African medicinal plants. 2nd ed. Boca Raton (FL): CRC Press/Taylor & Francis; 2014. https://doi.org/10.1201/b16292.
Johnson IS, Ettebong EO, Okokon JE, Johnson ES. Antipyretic potentials of ethanolic extract of Hilleria latifolia leaves in albino Wistar rats. Int J Herbal Med. 2018;6(6):50–53. Available from: https://www.florajournal.com/archives/2018/vol6issue6/PartA/6-3-3.1-717.pdf.
Dapaah G, Asante DB, Woode E, Agyare C, Abotsi WKM. Wound healing and cytotoxicity effects of Hilleria latifolia and Laportea ovalifolia. J Ethnopharmacol. 2017;202:265–73. https://doi.org/10.1016/j.jep.2017.03.009.
Woode E, Abotsi WKM, Mensah AY. Anxiolytic- and antidepressant-like effects of an ethanolic extract of the aerial parts of Hilleria latifolia (Lam.) H.Walter in mice. J Nat Pharm. 2011;2(2):62–71. https://doi.org/10.4103/2229-5119.83961.
Assob J.C.N., Kamga H.L.F., Nsagha D.S., Njunda A.L., Nde P.F., Asongalem E.A., Njouom R., Ada R.S. Antimicrobial and toxicological activities of five medicinal plant species from Cameroon traditional medicine. BMC Complement Altern Med, 2011; 11: 70. https://doi.org/10.1186/1472-6882-11-70.
Sofowora A. Medicinal plants and traditional medicine in Africa. 2nd ed. Ibadan: Spectrum Books Ltd; 1993. Available from: https://www.worldcat.org/oclc/31170917.
Trease GE, Evans WC. Pharmacognosy. 15th ed. London: Saunders; 2002. ISBN: 9780702026171.
Firdouse S, Alam P. Phytochemical investigation of the extract of Amorphophallus campanulatus tubers. Int J Phytomed. 2011;3(1):32–5. Available from: https://www.arjournals.org/index.php/ijpm/article/view/264.
Obuotor EM, Onajobi FD. Preliminary evaluation of the antioxidant properties of aqueous extract of Sorghum bicolor leaf sheath in mice. Phytother Res. 2000;14(8):608–10. https://doi.org/10.1002/1099-1573(200012)14:8
Ayinde BA, Omogbai EKI, Ikpefan EO. Comparative cytotoxic and antiproliferative effects of Persea americana Mill (Lauraceae) leaf, stem, and root barks. Niger J Pharm Sci. 2011;10:16–26.
Ikpefan EO, Ukwubile CA, Nwankwo LU. Cytotoxic, phytotoxic, and insecticidal assessment of the crude extract and fractions of leaves of Conyza sumatrensis (Retz.) E. Walker (Asteraceae). Niger J Pharm Appl Sci Res. 2020;9(3):52–8. Available from: https://www.nijophasr.com
Ayinde BA, Agbakwuru UC. Cytotoxic and growth inhibitory effects of the methanol extract of Struchium sparganophora Ktze (Asteraceae) leaves. Pharmacogn Mag. 2010;6(24):293–7. https://doi.org/10.4103/0973-1296.71795
Ikpefan EO, Ayinde BA. Comparative growth inhibitory assay of the methanol extract of the leaf and seed of Persea americana Mill (Lauraceae). J Pharmacogn Phytochem. 2013;1(6):101-107. Available from: https://www.phytojournal.com/archives/2013.v1.i6.74/comparative-growth-inhibitory-assay-of-the-methanol-extract-of-the-leaf-and-seed-of-persea-americana-mill-lauraceae
Ikpefan EO, Fajana A, Olowojoba JI. Cytotoxic and growth inhibitory effects of the methanol extract of Tridax procumbens Linn (Asteraceae). J Pharmacogn Phytochem. 2013;2(1):26-32. Available from: https://www.phytojournal.com/archives/2013/vol2issue1/PartA/4.pdf
Soltanian S, Fathi Najafi M, Mirshafiey A, Ghamarian A, Khorramizadeh MR, Abdollahi M. Cytotoxicity evaluation of methanol extracts of some medicinal plants on P19 embryonal carcinoma cells. J Appl Pharm Sci. 2017;7(7):142-149. doi: http://dx.doi.org/10.7324/JAPS.2017.70722
Hudec J, Gažarová M, Zajác P, Kobida L, Holková I, Mikušová L. In vitro cytotoxic effects of secondary metabolites present in Sarcopoterium spinosum. Appl Sci. 2021;11(11):5300. doi: http://dx.doi.org/10.3390/app11115300.
Elekofehinti OO, Kamdem JP, Bolingon AA, Ibrahim M, Sugiarto S, Adewumi AF. Saponins in cancer treatment: current progress and perspectives. Front Pharmacol. 2021; 12:680156. Doi: http://dx.doi.org/10.3389/fphar.2021.680156.
Ogundare OC, Adedosu T, Afolabi OK, Adeleke GE, Akinboro T, Daniel AA, Akoro S, Oludare VI. Ethnobotanical survey, physiochemical composition, and preliminary cytotoxic evaluation of some medicinal plants with anticancer potential from certain areas in South-West Nigeria. Ann Res Rev Biol. 2023;38(1):27-42. doi: http://dx.doi.org/10.9734/ARRB/2023/v38i130566.
Lim H, Lee SY, Ho LY, Sit NW. Mosquito larvicidal activity and cytotoxicity of the extracts of aromatic plants from Malaysia. Insects. 2023;14(6):512. doi: http://dx.doi.org/10.3390/insects14060512.
Adedokun O, Akinlo M, Adedeji I, Wande O, Ume O, Didacus N, Evans O. Unfolding the cytotoxic potential of Cassia siamea L. (Fabaceae) stem via a combination of cost-effective anticancer screening templates. Trop J Nat Prod Res. 2024;8(1):6056-6061. doi: http://dx.doi.org/10.26538/tjnpr/v8i1.50.
Ukwubile CA, Ahmed A, Katsayal UA, Yau J. Evaluation of preliminary cytotoxic and growth inhibitory effects of Melastomastrum capitatum (Vahl.) Fern. (Melastomataceae) leaf methanol extract by bench-top bio-assay. Int J Med Plants Nat Prod (IJMPNP). 2018;4(4):42-47. doi: http://dx.doi.org/10.20431/2454-7999.0404004.
Gbolade A, Muazu I, Haruna A. Comparative antioxidant, antiproliferative, and cytotoxic potentials of Piliostigma thonningii (Schum.) Milne-Redh. and Delonix regia (Boj. ex Hook) Raf. (Fabaceae) stem bark. Ethiop Pharm J. 2019;34(1):51-60. doi: http://dx.doi.org/10.4314/epj.v34i1.5
Sun W, Shahrajabian MH. Therapeutic potential of phenolic compounds in medicinal plants—Natural health products for human health. Molecules. 2023;28(4):1845. doi: http://dx.doi.org/10.3390/molecules28041845