Therapeutic effects of <I>Cyperus esculentus</I> on Monosodium glutamate-induced haemo-biochemical and steroidogenic anomalies in male Wistar rats
Main Article Content
Abstract
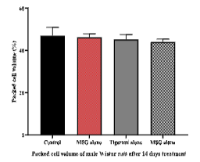
Monosodium glutamate (MSG) is a well-known food additive linked to anaemia and reproductive/hormonal disorders. The effects of Cyperus esculentus against MSG-induced toxicity in male animals have not been reported, therefore, the study aimed to investigatethe therapeuticeffects of Cyperus esculentus on MSG-induced haemo-biochemical and reproductive hormone anomalies in male rats. Forty adult male Wistar rats were randomly assigned into four groups (n=10). Group A served as the control, received 0.5 mL of distilled water orally. Group B, received MSG alone at 2 mg/kg orally, Group C received oral Cyperus esculentus at 500 mg/kg while Group D was treated with MSG alone orally at 2 mg/kg for the first 14 days, after which the rats received oral dose of Cyperus esculentus at 500 mg/kg for another 14 days.Five rats were sacrificed from each group 14 and 28 days post-treatment, respectively, during which samples were collected for haematological, biochemical and hormones assays. The packed cell volume and testosterone values in Group D were significantly higher than the values in Group B. It was concluded that Cyperus esculentus caused increased packed cell volume and testosterone, remarkable therapeutic effect on MSG-induced haematological and testosterone anomalies. Cyperus esculentus juice is therefore recommended as a potential haematinic and therapeutic agent for infertility in male rats exhibiting low libido.
Metrics
Article Details

This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License.
References
Oluwole DT, Ebiwonjumi OS, Ajayi LO, Alabi OD, Amos V, Akanbi G, Adeyemi WJ, Ajayi AF. Disruptive consequences of monosodium glutamate on male reproductive function: a review. Curr Res Toxicol. 2024; 6:100148. https//doi.org/10.1016/j.crtox.2024.100148.
Chakraborty SP. Patho-physiological and toxicological aspects of monosodium glutamate. Toxicol Mech Methods 2018; 29(6):389-396. https://doi.org/10.1080/15376516.2018.1528649.
Thuy LN, Salanta L, Tofana M, Socaci SA, Fărcaș AC, Pop CR. A mini review about monosodium glutamate. Bulletin UASVM Food Sci Technol. 2020; 77(1):1-12.
Helal EGE, Barayan AW, Abdelaziz MA, El-Shenawe NSA. Adverse effects of monosodium glutamate, sodium benzoate and chlorophyllins on some physiological parameters in male Albino rats. Egypt J. Hosp Med. 2019; 74(8):1857-1864.
Zanfirescu A, Unfurianu A, Tsatsaki AM, Nitulescu GM, Kouretas D, Veskoukis A, Tsoukals D, Engin AB, Aschner M, Margina D. A review of the alleged health hazards of monosodium glutame. Compr Rev Food Sci Food Saf. 2019; 18(4):1111-1134.doi:10.1111/1541-4337.12448.
Campbell A. Monosodium Glutamate. Encyclopedia of Toxicology (Third Edition), Wexler P. (Ed.). Academic Press, 2014; 391-392 p. https://doi.org/10.1016/B978-0-12-386454-3.00040-3
Mondal M, Tarafder P, Sarkar K, Nath PP, Paul G. Monosodium glutamate induces physiological stress by promoting oxygen deficiency, cell mediated immunosuppression and production of cardiovascular risk metabolites in rat. Int J. Pharm Sci Rev Res. 2014; 27(1):328-331.
Mukherjee I, Biswas S, Singh S, Talukdar J, Alqahtani MS, Abbas M, Nag TC, Mridha AR, Gupta S, Sharma JB, Kumari S, Dhar R, Karmakar S. Monosodium glutamate pertubs human trophoblast invasion and differentiation through a reactive oxygen species-mediated pathway: an in-vitro assessment. Antioxidants, 2023; 12: 634. https://doi.org/10.3390/antio12030634.
Kumar RN, Kumar PU, Hemalatha R. Monosodium Glutamate (MSG)-a food additive. Indian J. Nutr Diet. 2020; 98-107.
Hussin AM, Talaa AA. Fadhil S.A.N., Salman H.A. The adverse effect of long term intake of monosodium glutamate on kidney performance. Earth Environ Sci. 2021; 880:012056. Doi:10.1088/1755-1315/880/1/012056.
Bayram HM, Akgöz HF, Kızıldemir Ö, Öztürkcan SA. Monosodium glutamate: review on preclinical and clinical reports. Biointerface Res Appl Chem. 2023; 13(2): 149. https://doi.org/10.33263/BRIAC132.149
Shosha HM, Ebaid HM, Toraih EA, Abdelrazek HMA, Elrayess RA. Effect of monosodium glutamate on fetal development and progesterone level in pregnant Wistar-Albino rats. Environ Sci Pollut Res. 2023; 30:49779-49797. https://doi.org/10.1007/s11356-023-25661-x
Mustika D, Nishimura Y, Uneo S, Tominaga S, Shimizu T, Tajiri N, Jung C, Hida H. Central amygdala is related to the reduction of aggressive behavior by monosodium glutamate ingestion during the period of development in a ADHD model rat. Front Nutr. 2024; 11:1356189. Doi.10.3389/fnut.2024.1356189.
Rahimi Anbarkeh F, Baradaran R, Ghandy N, Jalali M. Nikravesh M.R., Soukhtanloo M. Effects of monosodium glutamate on apoptosis of germ cells in testicular tissue of adult rat: an experimental study. Int J. Reprod BioMed. 2019; 17(4):261-270. DOI: 10.18502/ijrm.v17i4.4551
Jubaidi FF, Mathialagan RD, Noor MM, Taib IS, Budin SB. Monosodium glutamate daily oral supplementation: study of its effects on male reproductive system on rat model. Syst Biol Reprod Med 2019; 65(3):194-204. https://doi.org/10.1080/19396368.2019.
Al-Khatawi GMG, Al-Attabi MRS, Bargooth AF. Physiological and histological study to the effects of monosodium glutamate in laboratory male rats and the protective role of vitamin E. Int J. Pharm Qual Assurance 2019; 10(2):272-279. Doi: 10.25258/ijpqa.10.2.10
Abdulghani MAM, Alshehade SA, Kamran S, Alshawsh MA. Effect of monosodium glutamate on serum sex hormones and uterine histology in female rats along with its molecular docking and in-silico toxicity. Heliyon, 2022; 8:e10967. https://doi.org/10.1016/j.heliyon.2022.e10967
Emmanuel NS, Bako IG, Malgwi IS, Tanko Y, Eze EO, Umar HA., Aliyu M, Muhammad A., Mohammed A. Preliminary monosodium glutamate-induced changes in mammary gland receptors and gene expression, water channel, oxidative stress, and some lactogenic biomarkers in lactating rats. J. Basic ApplZool. 2024; 85:3. https://doi.org/10.1186/s41936-024-00354-0
Sánchez‐Zapata E, Fernández‐López J, Angel Pérez‐Alvarez J. Tiger nut (Cyperus esculentus) commercialization: health aspects, composition, properties, and food applications. Compr Rev Food Sci Food Saf. 2012; 11(4):366-377.
Innih SO, Eluehike N, Francis B. Effects of aqueous extract of Cyperus esculentus (tiger nut) on antioxidant status and hematological indices in the heart of cadmium-induced Wistar rats. Nigerian J. Exp Clin Biosci. 2021; 9:17-2. DOI: 10.4103/njecp.njecp_32_20.
Edo GI, Onoharigho FO, Jikah AN, Oloni GO, Samuel PO, Rapheal OA, Ikpekoro O, Akpoghelie PO, Agbo JJ, Ekokotu HA, Ugbune U, Ezekiel GO, Abere GA, Oghroro EEA, Ojulari AE, Okoronkwo KA, Owheruo JO, Akpoghelie EO. Cyperus esculentus (tiger nut): An insight into its bioactive compounds, biological activities, nutritional and health benefits. Food Chem Adv. 2023; 3:100511. https://doi.org/10.1016/j.focha.2023.100511.
Lawal WS, Yusuf RT, Atanda AO, Hassan QO, Alaya AK, Olorundare TM. Organoleptic Characteristics of Tiger Nut Drink (Kunu Ayaya) Preserved By Herbs. Asian J. Food Res Nutr. 2023; 2(4):380–384. https://journalajfrn.com/index.php/AJFRN/article/view/62.
Nedviha S, Harasym J. Functional and Antioxidative Characteristics of Soft Wheat and Tiger Nut (Cyperus esculentus) Flours Binary Blends. Foods, 2024; 13(4):596. https://doi.org/10.3390/foods13040596.
Bazine T, Arslanoğlu F. Tiger nut (Cyperus esculentus); morphology, products, uses and health benefits. Black Sea J. Agric. 2020; 3(4):324-328.
Yu Y, Lu X, Zhang T, Zhao C, Guan S, Pu Y, Gao F. Tiger nut (Cyperus esculentus L.): nutrition, processing, function and applications. Foods, 2022; 11(4):601. Doi: 10.3390/foods11040601.
Kone B, Maiga M, Baya B, Sarro Y, Coulibaly N, Kone A, Diarra B, Sanogo M, Togo A, Goita D, Dembele M, Polis MA, Warfield J, Belson M, Dao S, Orsega S, Murphy RL, Diallo S, Siddiqui S. Establishing reference ranges of hematological parameters from malian healthy adults. J. Blood Lymph 2017; 7(1):154. doi: 10.4172/2165-7831.1000154.
Oyepata JS, Adekunle AT (2024). Haematological, Renal and Gastric Effects of Ethanol Leaf Extract of Bauhinia variegate Linn. Trop J. Phytochem Pharm Sci. 2024; 3(2):174–178. https://doi.org/10.26538/tjpps/v3i2.
Madu E, Wassagwa J, Ogbonnaya E, Adepoju OA, Elendu MU, Amoke VC. Comparative Anti-Anaemia Efficacy of atropha tanjorensis and Telfairia occidentalis Leaf Extracts in Aluminium Chloride (AlCl₃) Induced Anaemia in Albino Rats. Trop J. Phytochem and Pharm Sci. 2025; 3(9):461–464. https://doi.org/10.26538/tjpps/v3i9.6
Azghar A, Bensalah M, Berhili A, Slaoui M, Mouhoub B, El Mezgueldi I, Nassiri O, El Malki J, Maleb A, Seddik R. Value of hematological parameters for predicting patients with severe coronavirus disease 2019: a real-world cohort from Morocco. J. Int Med Res. 2022; 50(7):3000605221109381. doi: 10.1177/03000605221109381.
Sakr BR, Rabea RE, ElHamid SM. Value of hematological parameters as biomarkers of disease manifestations and severity in systemic sclerosis. Egyptian Rheumatol. 2021; 43(2):159-165. https://doi.org/10.1016/j.ejr.2020.06.008.
Waris A, Din M, Khalid A, Abbas Lail R, Shaheen A, Khan N, Nawaz M, Baset A, Ahmad I, Ali M. Evaluation of hematological parameters as an indicator of disease severity in Covid-19 patients: Pakistan's experience. J. Clin Lab Anal. 2021; 35:e23809. https://doi.org/10.1002/jcla.23809.
Huang B, Wang Z, Kong Y, Jin M, Ma L. Global, regional and national burden of male infertility in 204 countries and territories between 1990 and 2019: an analysis of global burden of disease study. BMC Public Health, 2023; 23:2195. https://doi.org/10.1186/s12889-023-16793-3.
Adejuyitan JA. Tiger nut processing: Its food uses and health benefits. Am J. Food Technol. 2011; 6(3):197–201. https://doi.org/10.3923/ajft.2011.197.201.
Igwebuike UM. The effects of oral administration of monosodium glutamaste (msg) on the testicular morphology and cauda epididymal sperm reserves of young and adult male rats. Vet. Arh. 2011; 81(4):525-534.
Udefa AL, Amama EA, Archibong EA, Nwangwa JN, Adama S, Inyang VU, Inyaka GUU, Aju GJ, Okpa S, Inah IO. Antioxidant, anti-inflammatory and anti-apoptotic effects of hydro-ethanolic extract of Cyperus esculentus L. (tigernut) on lead acetate-induced testicular dysfunction in Wistar rats. Biomed Pharmacother. 2020; 129:110491. Doi: 10.1016/j.biopha.2020.110491.
Coles EH. Veterinary clinical pathology. 4th Ed. W.B. Saunders Company, Philadelphia, London and Toronto:1986; 305- 326.
Meyer D, Harvey J. Veterinary Laboratory Medicine: Interpretation and Diagnosis, 2nd edition. Philadelphia: WB Saunders Co. 1998.
Ashaolu JO, Ukwenya VO, Okonoboh AB, Ghazal OK, Jimoh AAG. Effect of monosodium glutamate on hematological parameters in Wistar rats. Int J. Med Med Sci. 2011; 3(6):219-222. https://doi.org/10.5897/IJMMS.9000080
Ajibola M, Oloruntoba AC, Chinomso UA, Shekins O. The effects of orally administered monosodium glutamate (msg) on blood thrombocyte, blood coagulation and bleeding in rats. J. Pharm Biol Sci. 2012; 4(1):1-5.
ALhamed TA, Al-marzook FA, Al-Asady AM. The harmful effects of monosodium glutamate on blood parameters liver and kidney functions in adult white rats and the protective role of omega-3. Indian J. Forens Med Toxicol. 2021; 15(30):5245-5250.
Hassan HA. The potential effect of tigernut oil on some haemato-biochemical blood indices in male albino rats. Egypt J. Exp Biol. 2007; 3:49-54.
Enemali MO, Danielson EU, Bamidele TO. Effect of monosodium glutamate orally administered to male wister rats on some biochemical parameters. J. Biol Agric Healthcare, 2015; 5:24-28.
Nusaiba S, Fatima SA, Hussaini G, Mikail HG. Anaemogenic, Obesogenic and Thermogenic Potentials of Graded Doses of Monosodium Glutamate Sub-acutely Fed to Experimental Wistar Rats. Curr Clin Pharmacol. 2018; 13(4):273-278. doi: 10.2174/1574884713666181002120657
Abdel-Reheim ES, Abdel-Hafeez HA, Mahmoud BM, Abd-Allah EN. Effect of food additives (monosodium glutamate and sodium nitrite) on some biochemical parameters in albino rats. Int J. Bioassays, 2014; 3(08):3260-3273.
Al-Mousawi NH. Study on effect of glutamate monosodium exposure on some blood and biochemical parameters in adult albino rats. J. Entomol Zool Studies 2017; 5(6):1029-1031.
Sharma A. Monosodium glutamate-induced oxidative kidney damage and possible mechanisms: a mini review. Sharma J. Biomed Sci. 2015: 22:93. Doi 10.1186/s2929-015-0192-5.
Vyas N, Gamit K, Raval M. Male infertility: A major problem worldwide and its management in Ayurveda. Pharma Sci Monit. 2018; 9(1):446.
Abdou HM, Hassan EH, Aly RG. Monosodium glutamate (MSG): promoter of neurotoxicity, testicular impairment, inflammation and apoptosis in male rats. Swedish J. Sci Res. 2020; 1(2):78-90. DOI:10.51136/sjbsr.2020.78.90
Ekaluo UB, Ikpeme EV, Etta SE, Ekpo PB. Effect of aqueous extract of tigernut (Cyperus esculentus L.) on sperm parameters and testosterone level of male albino rats. Asian J.Biotechnol. 2015; 7(1):39-45.
Saad F, Gooren LJ, Haider A, Yassin A. A dose‐response study of testosterone on sexual dysfunction and features of the metabolic syndrome using testosterone gel and parenteral testosterone undecanoate. J. Androl. 2008; 29(1):102-105. Doi: 10.2164/jandrol.107.002774
Chiang HS, Hwang TIS, Hsui YS, Lin YC, Chen HE, Chen GC, Liao CH. Transdermal testosterone gel increases serum testosterone levels in hypogonadal men in Taiwan with improvements in sexual function. Int J. Impotence Res. 2007; 19(4):411-417.