Histopathology and Immunohistochemistry Evidence of Protective Effects of <i>Arachis hypogaea</i> Seed on 1, 2- Dimethylhydrazine-Induced Carcinogenesis in Male and Female Rat Colon
Main Article Content
Abstract
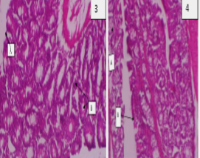
The study investigated the potential of a peanut-supplemented diet to reverse 1, 2- dimethyl hydrazine (DMH)-induced carcinogenesis linked tissue morphology and biomolecular alterations in rats’ colon. Fifty-six healthy Wistar rats of both sexes used for this study were divided into seven (7) groups of 4 rats each. Group A (control) rats were maintained on normal rat feeds. Groups B and C were maintained on normal rat feed and administered DMH (25 mg/kg body weight per week subcutaneously) for 12 and 24 weeks, respectively. After 12 weeks of DMH administration, a peanut-supplemented diet was provided for the remaining 12 weeks for Group D rats. Group E rats received DMH and a peanut-supplemented diet concomitantly for 24 weeks. Group F rats were on a peanut-supplemented diet for 12 weeks, followed by DMH administration for the remaining 12 weeks. Group G rats were maintained on a peanut-supplemented diet only for 24 weeks without DMH administration. At the end of the treatment period (24 weeks), the rats were sacrificed under mild anaesthesia, and portions of the colon were collected and fixed for histopathological and immunohistochemical examination. Colon histology revealed that DMH treatment without peanut supplementation caused severe histoarchitectural changes that were ameliorated by a peanut-supplemented diet. Immunohistochemistry of the colons showed that rats administered DMH only were positive for cytokeratin 20, indicating cancerous cells, and those exposed to DMH and peanut-supplemented diet were cytokeratin 20 negative. These results suggest that Arachis hypogaea seeds via peanut-supplemented diet protected rats’ colons against 1, 2-dimethylhydrazine-induced colon carcinogenesis.
Metrics
Article Details

This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License.
References
Irabor DO. Diet, environmental factors and increasing incidence of colorectal cancer in Nigeria. Ann Niger Med. 2014; 8:58-64.
Ntagirabiri R, Karayuba R, Niyonkuru S, Amani, M. Colorectal Cancer: Epidemiological, Clinical and Histopathological Aspects in Burundi. Open J. Gastroenterolo. 2016; 6: 83-87.
Tazinkeng NN, Pearlstein EF, Manda-Mapalo M, Adekunle AD, Monteiro JFG, Sawyer K, Egboh SC, Bains K, Chukwudike ES, Mohamed MF, Asante C, Ssempiira J, Asombang AW. Incidence and risk factors for colorectal cancer in Africa: a systematic review and meta-analysis. BMC Gastroenterol; 2024: 24: 303.
Irabor DO. Emergence of Colorectal Cancer in West Africa: Accepting the Inevitable. Niger Med J. 2017; 58(3):87-91.
Moen L, Liu B, Bukirwa P, Chingonzoh T, Chokunonga E, Finesse A, Korir A, Lamin B, Lorenzoni CF, Manraj SS, N'Da G, Odzebe AWS, Ogunbiyi O, Somdyala NIM, Packzowski, M, Parkin DM. Trends in the incidence of colorectal cancer in sub-Saharan Africa: A population-based registry study. Int J Cancer. 2024; 155(4): 675-682.
Ionescu VA, Gheorghe G, Bacalbasa N, Chiotoroiu AL, Diaconu C. Colorectal Cancer: From Risk Factors to Oncogenesis. Medicina (Kaunas). 2023;59(9):1646. doi: 10.3390/medicina59091646.
Gu H, Li B, Xiang L, Xu Z, Tang Y, Zhu Z, Jiang Y, Peng L, He H and Wang Y. Association between oxidative stress exposure and colorectal cancer risk in 98,395 participants: results from a prospective study. Front. Nutr. 2023; 10:1284066. doi: 10.3389/fnut.2023.1284066.
Terzic J, Grivennikov S, Karin E, Karin M. Inflammation and Colon Cancer. Gastroenterol. 2010; 138 (6):2101-2114.
Venkatachalam K, Vinayagam R, Arokia V, Anand M, Isa NM, Ponnaiyan R. Biochemical and Molecular Aspects of 1, 2-dimethylhydrazine (DMH)-induced colon carcinogenesis: A review. Toxicol Res (Camb). 2020; 9(1): 2 – 18.
Perše M, Cerar A. Morphological and molecular alterations in 1, 2-dimethylhydrazine and azoxymethane-induced colon carcinogenesis in rats. J Biomed Biotech. 2011; 10: 1155-1169
Perše M, Cerar A. The dimethylhydrazine induced colorectal tumours in rat - experimental colorectal carcinogenesis. Radiol Oncol. 2005; 39(1): 61-70.
Ogbeide OK, Akhigbe IU, Unuigbe CA, Erharuyi O, Imieje V, Benjamin GO, Ikeke K, Ayeni B, Irabor E, Owolabi JB, Falodun A. Histological assessment and Antimicrobial Investigation of Pure Compound; 3,5,6,7-tetrahydroxy-19-vouacanoic acid;(3β, 5α, 6β, 7β)- form,6,7-dibenzoyl (pulcherrimin A) Isolated from Caesalpinia pulcherrima Stem Bark. Trop J Phytochem Pharm. Sci. 2023; 2(1):30-37.
Uneyama M, Chambers JK, Nakashima K, Uchida K, Nakayama H. Histological Classification and Immunohistochemical Study of Feline Colorectal Epithelial Tumors. Vet Pathol. 2021;58(2):305-314.
Fleming M, Ravula S, Tatishchev SF, Wang HL. Colorectal carcinoma: Pathologic aspects. J Gastrointest Oncol. 2012 ;3(3):153-173.
Dunn C, Brettle D, Cockroft M, Keating E, Revie C, Treanor D. Quantitative assessment of H&E staining for pathology: development and clinical evaluation of a novel system. Diagn Pathol. 2024; 19 (42): 1-11. https://doi.org/10.1186/s13000-024-01461-w
Kabiraj A, Gupta J, Khaitan T, Bhattacharya PT. Principle and techniques of immunohistochemistry review. Int J Biol Med Res. 2015; 6(3): 5204-5210.
Al-Maghrabi J, Emam E, Gomaa W. Immunohistochemical staining of cytokeratin 20 and cytokeratin 7 in colorectal carcinomas: Four different immunostaining profiles. Saudi J Gastroenterol. 2018; 24(2):129-134.
Harbaum L, Pollhesimer M.J., Kornprat P, Lindtner RA, Schlemmer A, Rehak P, Langner C. Keratin 20- a diagnostic and prognostic marker in colorectal cancer? Histol. Histopathol. 2012; 27: 347-356.
Vernia F, Longo S, Stefanelli G, Viscido A, Latella G. Dietary Factors Modulating Colorectal Carcinogenesis. Nutrients. 2021 ;13(1):143. doi: 10.3390/nu13010143.
Mohammad NMAB, Shahril MR, Shahar S, Fenech M, Sharif R. Association between Diet-related Behaviour and Risk of Colorectal Cancer: A Scoping Review. J Cancer Prev. 2022;27(4):208 -220.
Baena R, Salinas P. Diet and colorectal cancer. Maturitas. 2015 Mar;80(3):258-64. doi: 10.1016/j.maturitas.2014.12.017.
World Cancer Research Fund (WCRF). Third Expert Report, Diet, Nutrition, Physical Activity, and Cancer: a Global Perspective, Continuous Update Expert Report. 2018 https://www.wcrf.org/dietactivity-and-cancer/global-cancer-update-programme/about-the-thirdexpert-report/
Costea T, Hudiță A, Ciolac OA, Gălățeanu B, Ginghină O, Costache M, Ganea C, Mocanu MM. Chemoprevention of Colorectal Cancer by Dietary Compounds. Int J Mol Sci. 2018;19(12):3787. doi: 10.3390/ijms19123787.
Hu R, Chantana W, Pitchakarn P, Subhawa S, Chantarasuwan B, Temviriyanukul P, Chewonarin T. Ficus dubia latex extract prevent DMH-induced rat early colorectal carcinogenesis through the regulation of xenobiotic metabolism, inflammation, cell proliferation and apoptosis. Sci Rep. 2022;12(1):15472. doi: 10.1038/s41598-022-19843-9.
Shojaei-Zarghani S, Rafraf M, Khosroushahi AY, Sheikh-Najafi S. Effectiveness of theobromine on inhibition of 1,2-dimethylhydrazine-induced rat colon cancer by suppression of the Akt/GSK3β/β-catenin signalling pathway. J. Funct. Foods; 2020: 75. https://doi.org/10.1016/j.jff.2020.104293.
Ikponmwosa-Eweka O, Olorunyomi JS, Ezeuko VC, Oriakhi K. Apoptotic and antioxidant activities of extracts of Spondias monbin stem bark in dimethylhydrazine (DMH)-induced colorectal carcinogenesis in Wistar rats. Phytomed. Plus. 2023; 3(4):1-11.
Engel N, Oppermann C, Falodun A, Kragl U. Proliferative effects of five traditional Nigerian medicinal plant extracts on human breast and bone cancer cell lines. Journal of Ethnopharmacology. 2011; 37 (2):1003-1010. https://doi.org/10.1016/j.jep.2011.07.023
Mentella MC, Scaldaferri F, Ricci C, Gasbarrini A, Miggiano GAD. Cancer and Mediterranean Diet: A Review. Nutrients. 2019 Sep 2;11(9):2059. doi: 10.3390/nu11092059.
Rossi YE, Bohl LP, Vanden Braber NL, Ballatore MB, Escobar FM, Bodoira R, Maestri DM, Porporatto C, Cavaglieri LR, Montenegro MA. Polyphenols of peanut (Arachis hypogaea L.) skin as bioprotectors of normal cells. Studies of cytotoxicity, cytoprotection, and interaction with ROS. J. Funct. Foods. 2020; 67:103862. https://doi.org/10.1016/j.jff.2020.103862
Isoje AO, Obi FO. Nutritional and Phytochemical components and in vitro antioxidant capacity of raw Arachis hypogaea seeds. J. Med. Plants Stud. 2021. 9(2): 35-39.
Animal Care and Use Program. In Guidelines for Care and Use for Scientific Research. (8th Ed), National Academic Press, Washington. 2011
Veceric Z, Cerar A. Comparison of Wistar vs. Fischer rat in the incidence of 1, 2-dimethylhydrazine induced intestinal tumours. Radiol Oncol. 2004; 38: 227-34.
Bancroft, JD, Gamble M. Theory and Practice of Histological Techniques (6th ed.). Churchill Livingstone, London. Elsevier. China. 2008
Perry C, Chung JY, Ylaya K, Choi CH, Simpson A, Matsumoto KT, Smith WA, Hewitt SM. A Buffered Alcohol-Based Fixative for Histomorphologic and Molecular Applications. J Histochem Cytochem. 2016 ;64(7):425-40.
Bailey SA, Zidell RH, Pery RW. Relationship between organ weight and body/brain weight in the rat: what is the best analytical endpoint? Toxicol. Pathol. 2004; 32:448- 466.
Fernandes T, Oliveira MI, Castro R, Araújo B, Viamonte B, Cunhaet R. Bowel wall thickening at CT: simplifying the diagnosis. Insights Imaging. 2014; 5:195–208. https://doi.org/10.1007/s13244-013-0308-y
Thangaraj K, Natesan K, Palani M, Vaiyapuri M. Orientin, a flavonoid, mitigates 1, 2 dimethylhydrazine-induced colorectal lesions in Wistar rats fed a high-fat diet. Toxicol Rep. 2018;5: 977-987.
Guyton M, Verghese M, Walker LT, Chawan CB, Shackelfold L, Jones J, Kwatiwada J. Inhibitory Effects of Feeding Selected Levels of Peanuts on Axoxymethane-Induced Aberrant Crypt Foci in Male Fisher 344 Rats. Int. J Cancer Res. 2008; 4(4): 146-143.
Reid HM, Sunkara R, Shackelford L, Walker LT, Verghese M. Feeding Walnuts and Peanuts Reduced Development of Azoxymethane-Induced Precancerous Lesions. Food and Nutr Sci. 2016; 7: 440-446
Sengottuvelan M, Nalini N. Dietary supplementation of resveratrol suppresses colonic tumour incidence in 1, 2-dimethylhydrazine-treated rats by modulating biotransforming enzymes and aberrant crypt foci development. British J Nutri. 2006; 96:145–153
Boivin GP, Washington K, Yang K, Ward JM, Pretlow TP, Russell R, Besselsen DG, Godfrey VL, Doetschman T, Dove WF, Pitot HC, Halberg RB, Itzkowltz SH, Groden J, Coffey RJ. Pathology of mouse models intestinal cancer: consensus report and recommendations. Gastroenterol. 2003; 124(3): 762-777.
Jucá MJ, Bandeira BM, Carvalho DS, Leal AT. Comparative study of 1,2-dimethylhydrazine and azoxymethane on the induction of colorectal cancer in rats. J. Coloproctol. 2013; 34(3): 167-173
Maru GB, Kumar G. Ghantasala S, Tajpara P. Polyphenol-Mediated In Vivo Cellular Responses during Carcinogenesis. In: Polyphenols in Health and Diseases. Ronald RW, Victor RP, Sherma Z. (eds). Elsevier. 2014; 2:1141-1179.
Isoje AO, Obi FO. Arachis Hypogeae Seed Powder Ameriorated 1, 2-Dimethylhyydrazine-Induced Oxidative Stress in Exposed Rats. Scientia Africana. 2023; 22(3): 107-114.
Zinov’eva VN, Spasov AA. Mechanisms of plant polyphenols anticancer effects. I. Blockade of carcinogenesis initiation. Biomed Khim. 2012; 58 (2):160-175.
Barzi A, Lenz AM, Labonte MJ, and Lenz HJ. Molecular Pathways: Estrogen pathway in colorectal cancer: Clin Cancer Res. 2013 19: 2.
Hartman J, Gustafsson J. Estrogen Receptors in Colorectal Cancer: Goalkeepers, Strikers or Bystanders? Cancer Prev Res. 2010; 3(8): 897-899.
Abdulkadir WS, Puana F, Taupik M, Tungadi R, Hutuba AH, Djuwarno EN, Ramadhani FN, Hiola F. In Silico Analysis of Isoflavone Compounds in Soybean (Glycine max L) as Anti-Breast Cancer Agents Targeting Estrogen Receptor Alpha. Trop J. Phytochem Pharm. Sci.2024;3(7): 375 -379. http://www.doi.org/10.26538/tjpps/v3i7.3.
Nilsson S, Makela S, Treuter E, Tujague M, Thomsen J, Andersson G. Mechanisms of estrogen action. Physiol. 2021; 81:1535–1565.
Sipos F, Műzes G. Isolated lymphoid follicles in colon: Switch points between inflammation and colorectal cancer? World J Gastroenterol. 2011; 17(13): 1666-1673.
Nabil HM, Hassan BN, Tohamy AA, Waaer HF, Monei AE. Radioprotection of 1, 2-dimethylhydrazine-initiated colon cancer using low-dose γ rays by modulating multidrug resistance-1, cytokeratin 20, and β-catenin expression. Hum. Exp. Toxicol. 2016; 35(3): 282-292.
Elkareem AW, Mohameed SO. Immunohistochemical expression of cytokeratin 20 in colorectal cancer. Eur Academic Res. 2016; 3(2): 11683-11691.
Rullier A, Le BB, Fawaz R. Cytokeratin 7 and 20 expressions in cholangiocarcinomas varies along the biliary tract but still differs from that in colorectal carcinomas metastasis. Am. J. Surg. Pathol. 2000. 24: 870 - 876.
Park SY, Kim BH, Kim JH. Panels of immunohistochemical markers help determine primary sites of metastatic adenocarcinoma. Arch. Pathol. Lab. Med. 2007; 131: 1561–1567